Too often, Sterile Processing technicians are faced with multiple directives about how to ideally process a medical device. As required by law, manufacturers of medical devices, biological indicators, chemical indicators, sterility maintenance systems (packaging) and sterilization equipment are required to produce Instructions for Use (IFUs) in order to obtain a 510K FDA. clearance for the optimal use of their products. Through no fault of these manufacturers, these instructions don’t always line up, primarily because of the many legitimate ways their products can be processed or used in sterilization procedures.
Competing directives from these manufacturers can put an SP technician in a situation known as “resolving multiple IFUs.” These healthcare professionals, who are looking for definitive answers, face this all too often as they carry out their critical functions.
The challenge of resolving multiple IFUs
Manufacturers’ IFUs are the gold standard for device processing guidance and they’re universally accepted. ANSI, AAMI, ISO and other sources of best practice information appropriately point back to IFUs.
The challenge is that a technician must refer not only to an IFU for a specific medical device but also the IFU for the biological and chemical indicators, the sterilizing medical equipment they use (e.g., an autoclave or a steam sterilizer) and still another IFU for the sterility maintenance system they utilize (e.g., blue wrap, peel pouches, and rigid containers).
Addressing the multiple-IFU scenario
AAMI (Association for the Advancement of Medical Instrumentation) is methodically working to develop guidance on the issue of resolving multiple IFUs. In the ANSI/AAMI ST79:2017 Instrumentation Standards, the organization took the first step and stated essentially that when there’s an unresolvable conflict between a sterilizer IFU and a device IFU, SP technicians should follow the device IFU exposure directives.
AAMI also has advised that in the case of an unresolvable conflict regarding cycle parameters between a container system IFU and a sterility maintenance system IFU, the directives from the container system manufacturer should be followed.
Interim guidance
So far so good. But AAMI recognizes there are many additional conflicting IFU scenarios that still need to be addressed. I believe this will happen in the next revision of the ANSI/AAMI ST79 Standard.
In the meantime, AAMI advises SPD teams to conduct a risk assessment to determine how their facility will resolve these situations. They should do a side-by-side analysis of the pertinent IFUs as part of risk analysis for each scenario and develop robust policies and procedures, utilizing recognized industry guidelines, for the following:
- Storage/Shelf life
- Definition of “Immediate Use”
- Sterilization user verification, to include the sterilizer, medical device(s) and sterile barrier system
- Utilization of IFUs for reprocessing activities. It’s important to note that cleaning, sterilization and drying/storage are distinctly different processes and thus require different IFU usage. The table below outlines typical Validated IFU Labeling Claims.
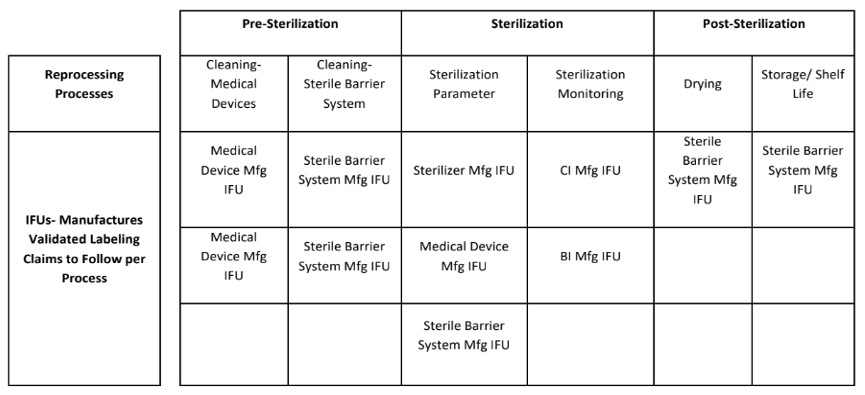
My own 50,000-foot risk assessment recommendations
Specifically, when it comes to risk assessments for sterility maintenance systems, I suggest SPD teams keep in mind that when device manufacturers submit their test data, the FDA requires them to test just one packaging system, and far more often than not, these manufacturers only test using the blue wrap sterility maintenance system. Their IFUs, therefore, can call for as much as one hour of drying time after the sterilization process, even though there are packaging systems and immediate use scenarios that the manufacturers haven’t addressed in their tests that require no drying time at all.
Therefore, a good unofficial rule of thumb from my perspective is to default to the medical device IFU for cleaning and sterilization guidance and to the sterility maintenance system IFU for guidance regarding drying time. But again, each facility should clarify these murky areas for themselves based on manufacturer information and their own informed expertise.
As a member of the AAMI ST79 working group that’s preparing to address the next revision to that document, I can assure you that I and my colleagues will do our best to clarify all possible conflicting IFU scenarios to help ensure safe, efficient device processing. An even better scenario would be if more users join us in the upcoming revision. We need your help – and after all, you are the experts.
David Jagrosse, CHL, CRCST, President of David Jagrosse Consulting LLC, is a consultant to oneSOURCE. David is past President of IAHCSMM and currently serves on the ANSI/AAMI ST79 Working Group. The views expressed here are his own and are not made on behalf of any other organization.





